ProductKANEKA Direct RT-PCR kit SARS-CoV-2(IVD)
This kit is designed for the detection of SARS-CoV-2 in approximately one hour by employing our proprietary simple pretreatment method and One step RT-qPCR method. This product is an in vitro diagnostic product.
(Approval number:30300EZX00058000)
Features
- No need for RNA purification (samples: nasopharyngeal swabs and saliva)
- No channel required for Cy5 detection (FAM and ROX are used for detection)
- Simultaneous detection of internal control (internal standard)
<Protocols>
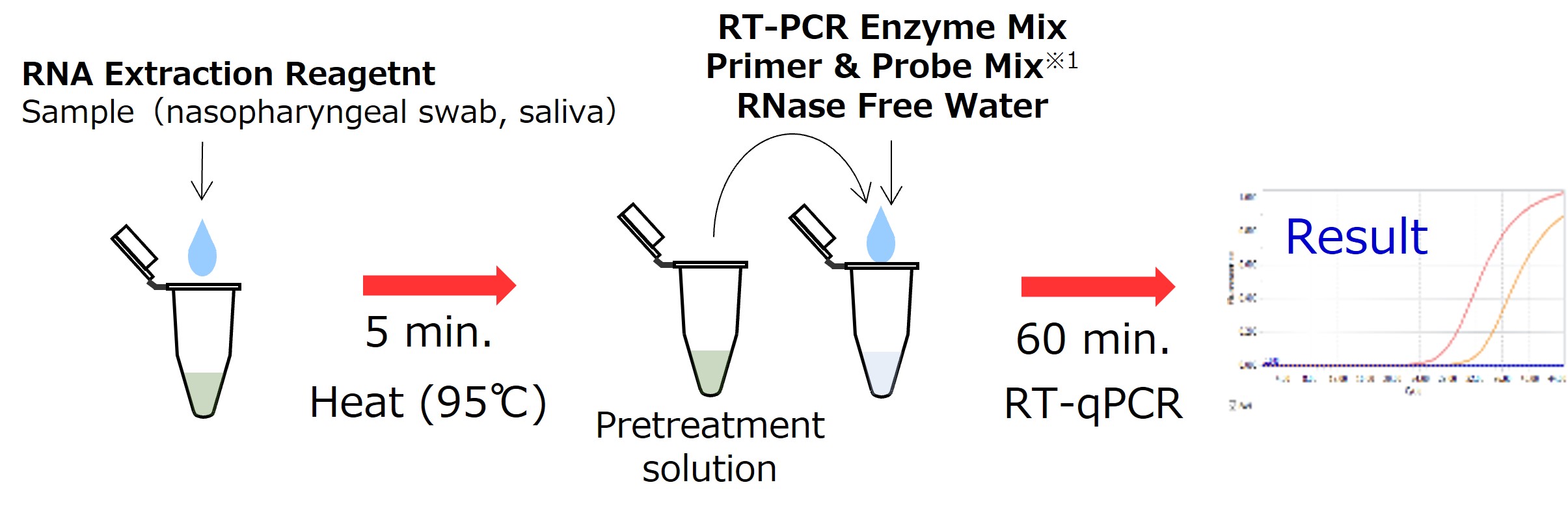
* 1 Primer & Probe Mix uses the sequences (targets: N1, N2) described in the following by the US CDC. 2019-Novel Coronavirus(2019-nCoV) Real-time RT-PCR Panel Primers and Probes (Effective : 24 Jan 2020)
* 2 The performance of this kit has been confirmed by the following real-time PCR devices.
* 3 Add when using a real-time PCR device that requires ROX correction.
This testing kit was validated in a government report, “Validation of 2019-nCoV gene testing method in which evaluation results were obtained using clinical specimens”,(published by the National Institute of Infectious Diseases and the Tuberculosis and Infectious Diseases Control Division of the Ministry of Health, Labor and Welfare of Japan), and is covered by public medical insurance in Japan.
<Evaluation data of this kit>
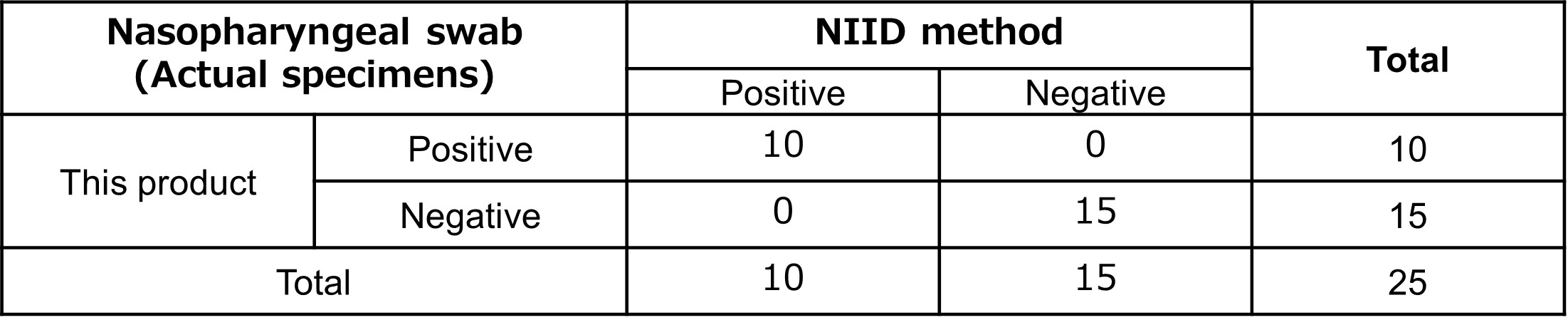
Clinical performance test results (1)
By using positive specimens from 10 patients and negative specimens from 15 patients, the product was compared with the RNA purification method described in the "Manual for the Detection of Pathogen 2019-nCoV Ver. 2.9.1"2), issued by the National Institute of Infectious Diseases. The comparison test showed a positive agreement rate of 100% and a negative agreement rate of 100%, with a total agreement rate of 100%.
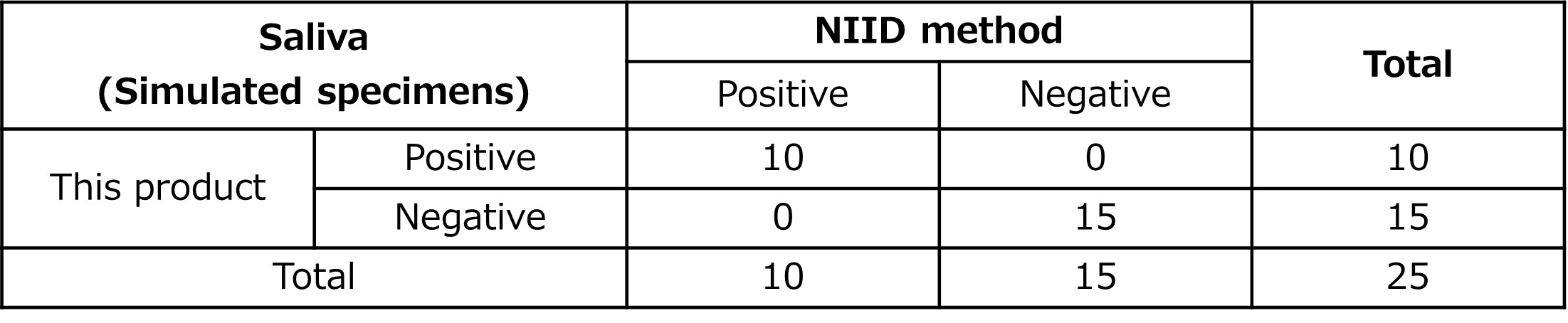
Clinical performance test results (2)
Positive specimens from nasopharyngeal swabs were spiked into negative salivary specimens to prepare simulated specimens. Using these simulated specimens, the product was compared with the RNA purification method described in the "Manual for the Detection of Pathogen 2019-nCoV Ver. 2.9.1"2), issued by the National Institute of Infectious Diseases. The comparison test showed a positive agreement rate of 100% and a negative agreement rate of 100%, with a total agreement rate of 100%.
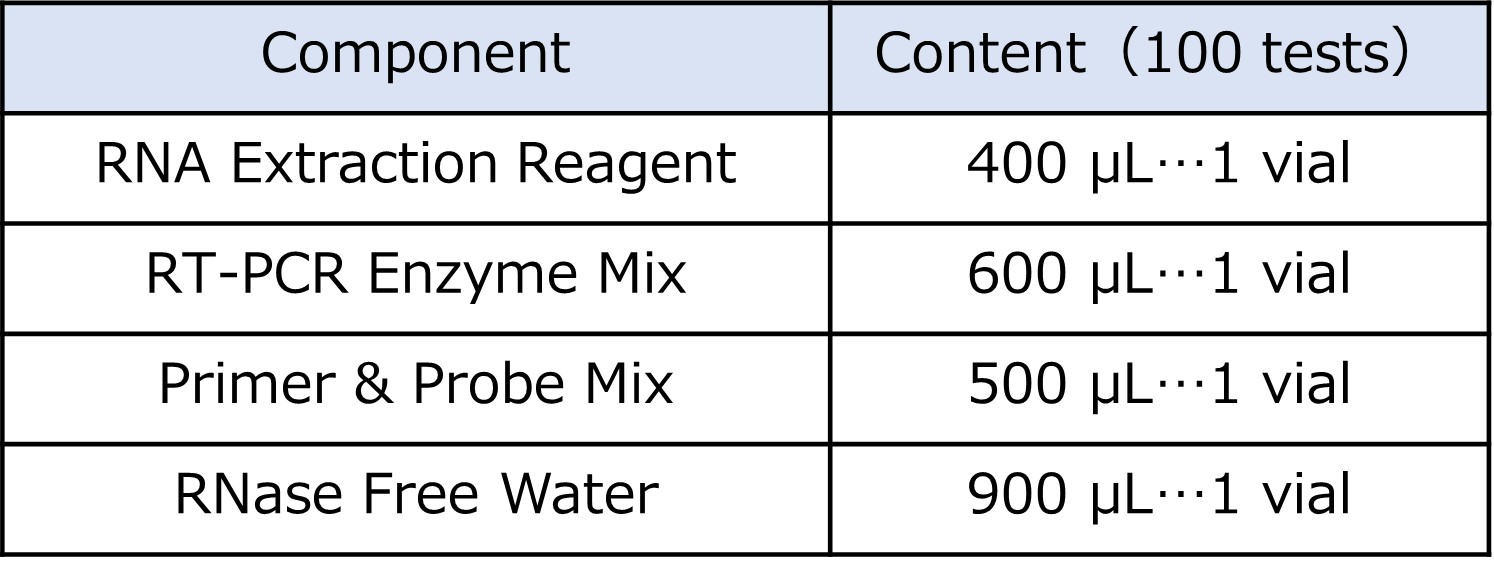
<Components of the Kit>
<Product Information>
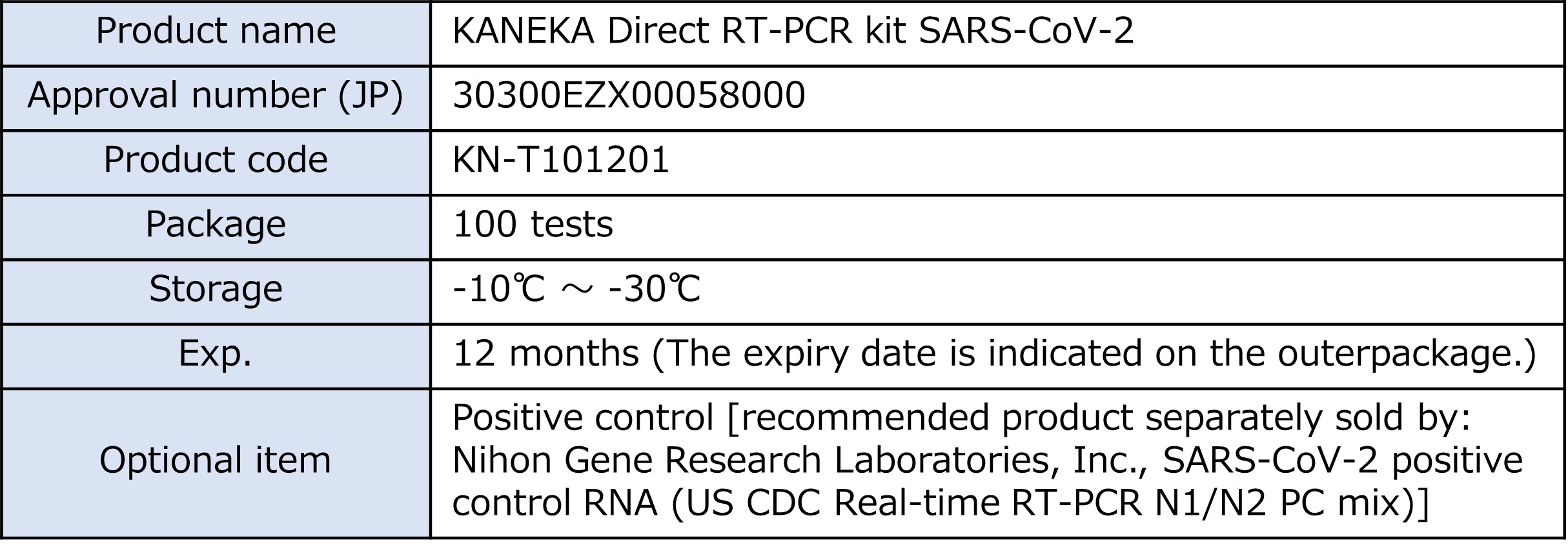
・Storage Temperature: Below -20℃
・Expiration Date:1 year from manufacturing
>>Click here for Instruction for Use(June 2021 (1st version))
【Important Basic Precautions】
- Negative results from this test do not preclude SARS-CoV-2 infection.
- Results from this test should not be used as the sole basis to diagnose SARS-CoV-2 infection; infection status should be determined comprehensively by including the patient's clinical symptoms.
Refer to the updated information for medical institutions and inspection agencies released by the Ministry of Health, Labour and Welfare. - Use the necessary biohazard measures when collecting and handling specimens.
- For specimens to be used for testing, please refer to the "Guidelines for Novel Coronavirus Infection (COVID-19) Pathogen Testing" published by the Ministry of Health, Labour and Welfare.
>>We are seeking distributors all over the world, please contact us.