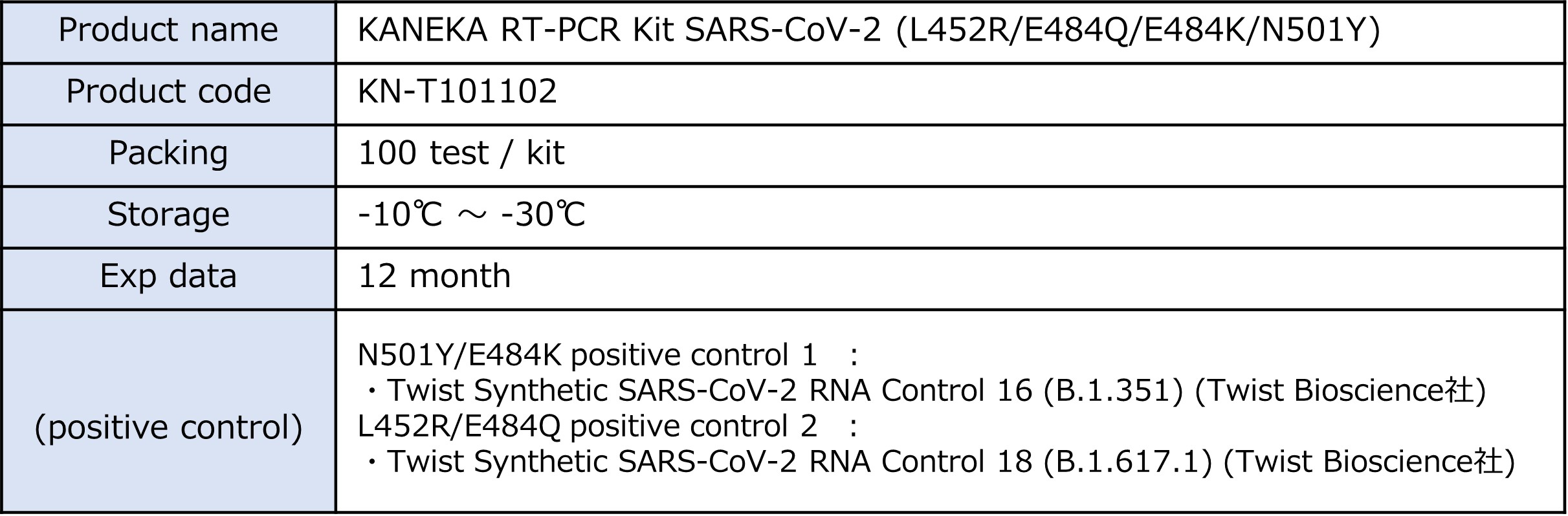
ProductKANEKA RT-PCR Kit “SARS-CoV-2(L452R/E484Q/E484K/N501Y)”
<Main Features>
- It allows the simultaneous detection of four mutations*1 in the spike protein (N501Y, E484K, E484Q and L452R mutations) in a single PCR test.
- Operation step: (Sample collection → RNA extraction) → One-step RT-qPCR (about 1 hour)
- Applicable samples: Nasopharyngeal swab, saliva
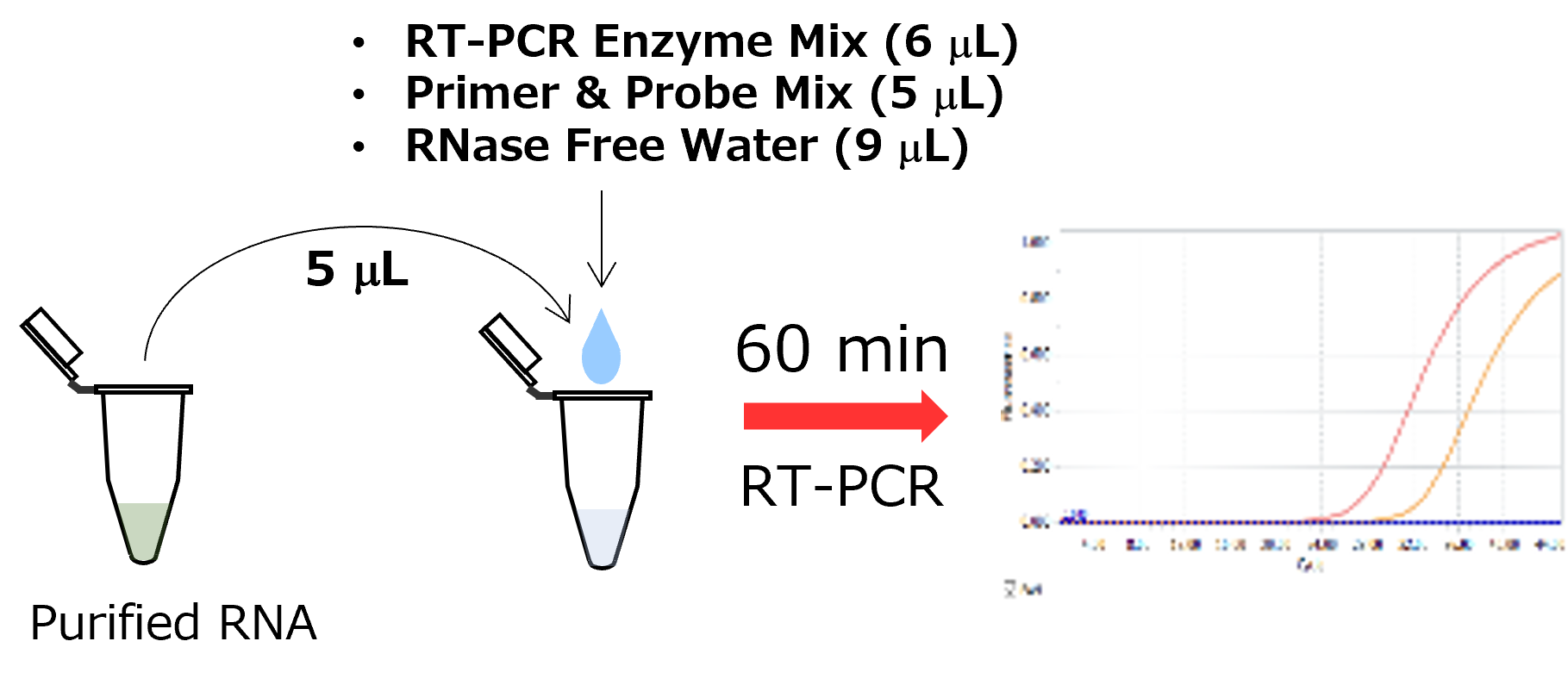
<Protocols>
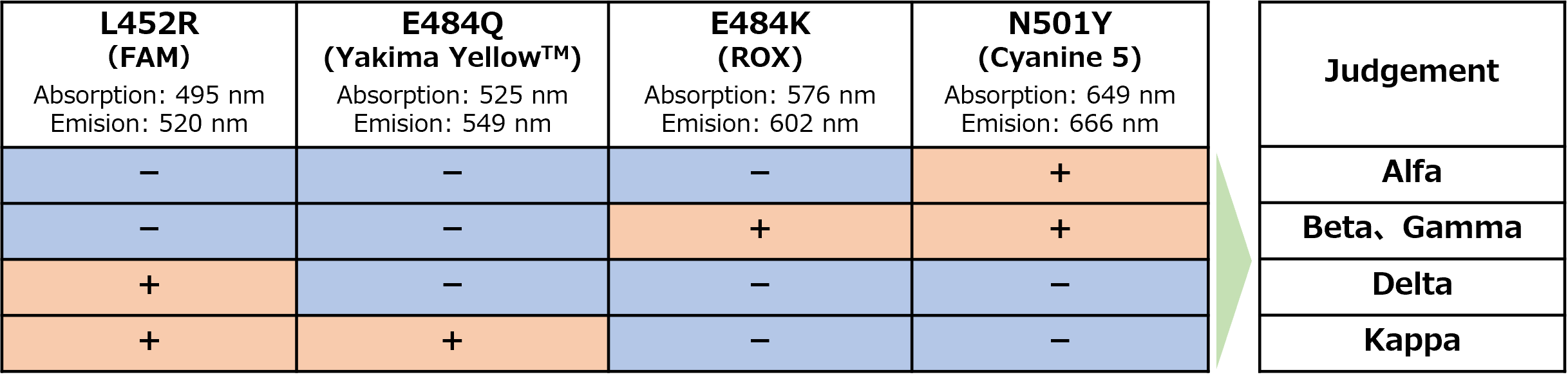
The performance of this product has been verified on the following real-time PCR devices.
If other devices are used, the validity of the detection results should be verified by the customer.
- LightCycler® 96 System (Roche Diagnostics)
- LightCycler® 480 System II (Roche Diagnostics)
- QuantStudio 3 Real-Time PCR System (96well,0.2 mL block) (Thermo Fisher Scientific社)
※"CronoSTAR" is a trademark of Takara Bio Inc.
※"LightCycler"is a trademark of Roche Diagnostics Ltd.
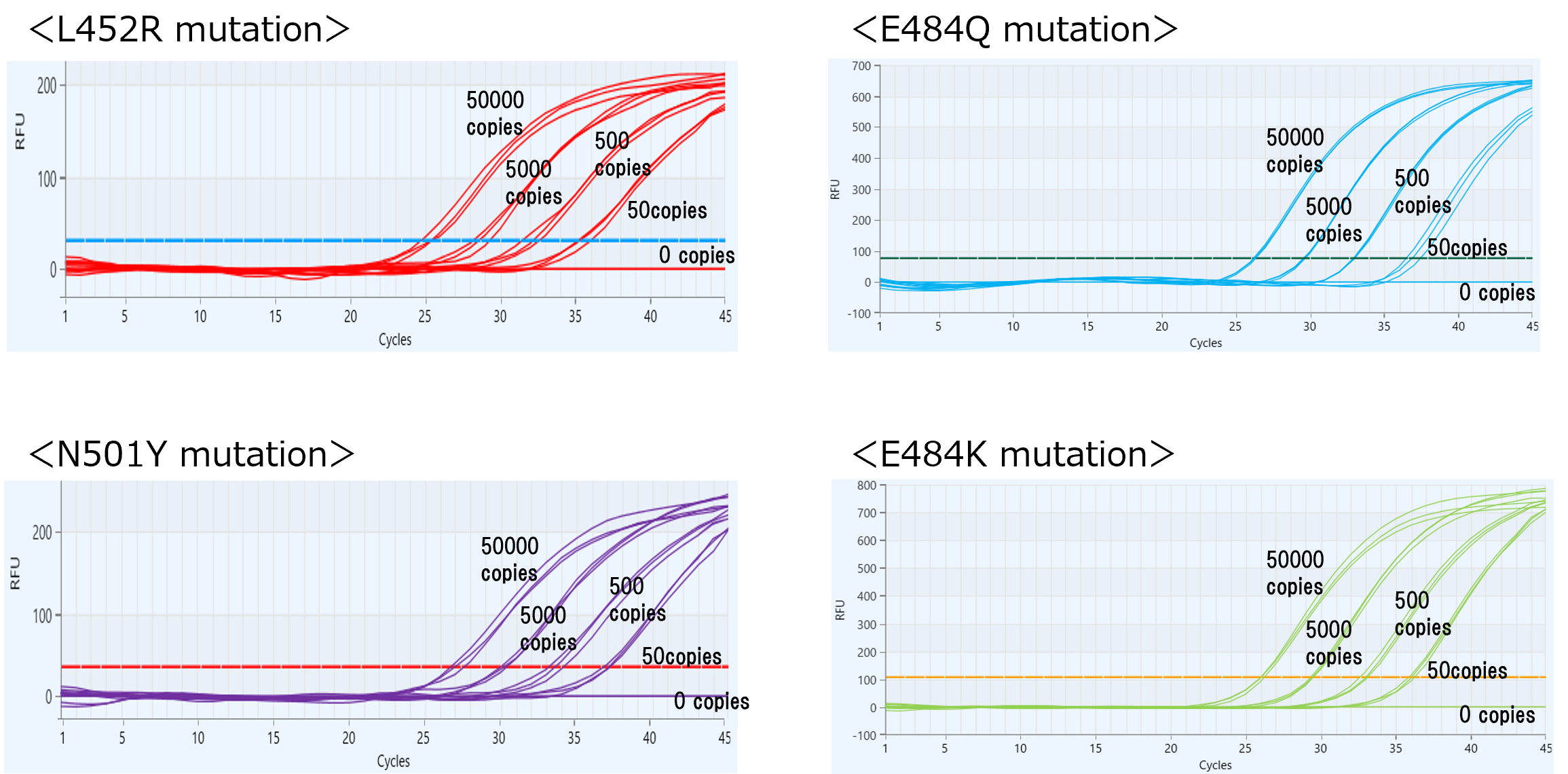
<Evaluation data of this kit>
<Test Example> (Instrument:CronoSTAR® 96 Real-Time PCR System(Takarabio USA))
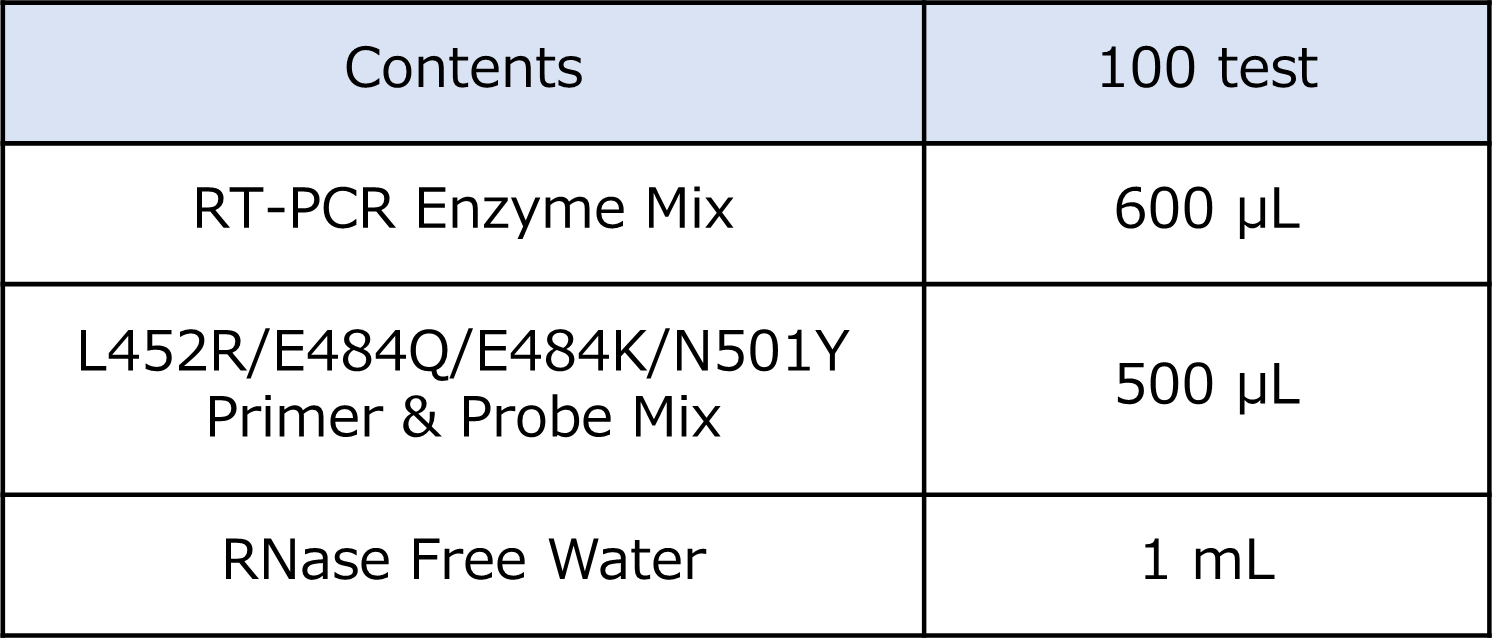
<Components of the Kit>
<Product information>
>>Click here for product manual